Milk contains both whey and micellar casein protein (~20 and 80 % of total protein content, respectively). Whey is a fast digesting protein. In contrast, micellar casein is a slow digesting protein (Gorissen et al, 2020).
Micellar casein clots in the stomach due to the acidic environment and the presence of pepsin. However, the micellar casein structure can also be intentionally disrupted by various food processing techniques, resulting in food products such as cheese and yoghurt.
In addition, micellar casein can be processed to caseinate. Caseinate is more soluble than micellar casein and is believed to be more rapidly digested and absorbed. However, micellar casein and caseinate have never been directly compared.
Caseinate protein can be further processed by cross-linking enzymes to change properties such as its firmness and viscosity. However, little is known about the digestion and absorption of cross-linked caseinate.
Therefore, we designed a study to compare the plasma amino acid response following the ingestion of micellar casein, caseinate, and cross-linked caseinate.
We recruited 15 healthy young males to participate in a randomized cross-over study (i.e. each subject underwent 3 experimental test days, once for each protein drink)
The subjects received 40 g of one of protein drinks and we took repeated blood samples over a 6 h period.
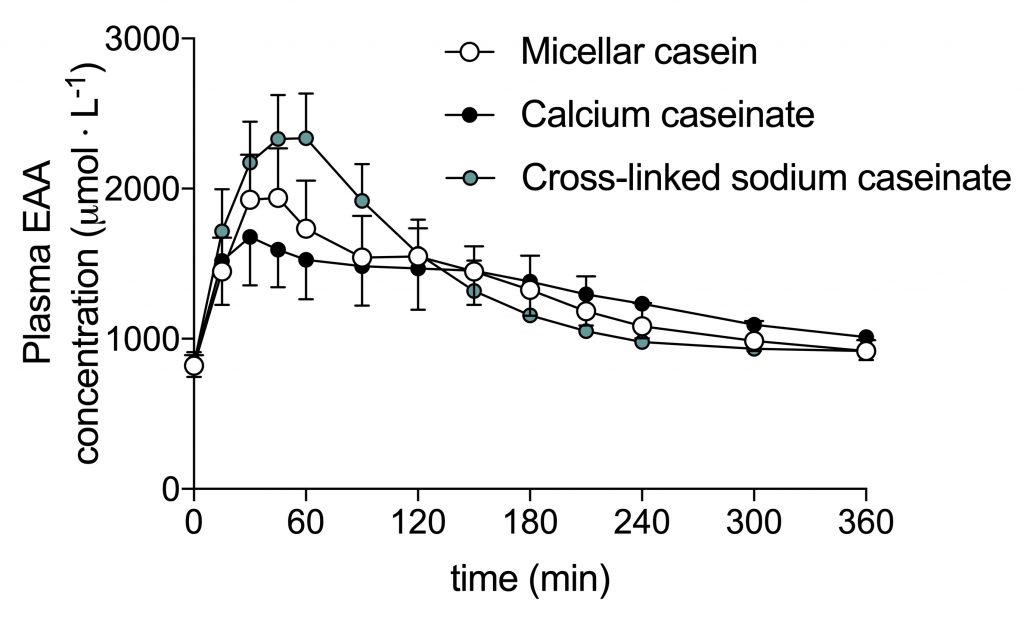
To our surprise, the micellar casein appeared to be more rapidly digested and absorbed than the (calcium) caseinate.
Micellar casein is classified as a slowly digestible protein, but it appeared to be digested and absorbed pretty rapidly in this study. In fact, when you look at the scientific literature, you see huge differences in the digestion and absorption speed of casein: fast, slow, and everywhere in between.
There’s no clear explanation for the variation seen in micellar casein digestion. However, it has been reported that micellar casein products vary drastically in their solubility. Therefore, we measured the solubility of our micellar casein, and found it was surprisingly low. This low solubility might impact the casein clotting, and therefore its digestion speed.
Therefore, protein solubility might be an important determinant of (casein) protein digestion. But this remains to be established in studies that are designed to answer that question.
The digestion and absorption of our (calcium) caseinate protein was slower than we expected. This may be related to the specific salt-form of caseinate we used (i.e. calcium caseinate).
There is some evidence that suggests that calcium increases the clotting of casein proteins based on experimental digestion models. Therefore, calcium caseinate might digest slower than sodium caseinate.
The cross-linked sodium caseinate appeared to be the most rapidly digesting protein. Only one prior study so far has looked at the impact of crosslinking on protein digestion, but found no effect of protein cross-linking. We think the reason that the cross-linked sodium caseinate was so fast is because it was the sodium form of caseinate, not because of the cross-linking.
So what have we learned from this study?
The main lessons are that not all casein protein is equal, and that small changes in the production and/or storage of (casein) protein might drastically impact its behavior.
Our data suggest that the salt-form of caseinate might be relevant for its digestion and absorption.
In addition, our data suggest that the solubility of (casein) protein may be a relevant factor that modulates its digestion and absorption.
We have previously shown that hydrolyzation of micellar casein increases its digestion and absorption. In addition, we have shown that glycation of milk protein reduces the release of the amino acid lysine into the circulation (Nyakayiru et al, 2020).
The duration and conditions of protein storage can also impact its functional solubility and its glycation level (and probably many more things).
Therefore, there is a long list of food processing and storage factors that can impact the behavior of (casein) protein. Clearly, there is a need to:
- Better understand the impact of various food processing and storage factors
- Better standardization and/or documentation of these factors so that specific products behave as expected and consistently.
Our OPEN-ACCESS study:
Trommelen et al. Casein protein processing strongly modulates post-prandial plasma amino acid responses in vivo in humans. Nutrients, 2020


Leave a Reply