Our new review is now online!
Note that this was a methodological review and does not have many practical applications for those who want to optimize protein synthesis. For more practical advice, see our comprehensive blog article The Ultimate Guide to Muscle Protein Synthesis, or our scientific review (1).
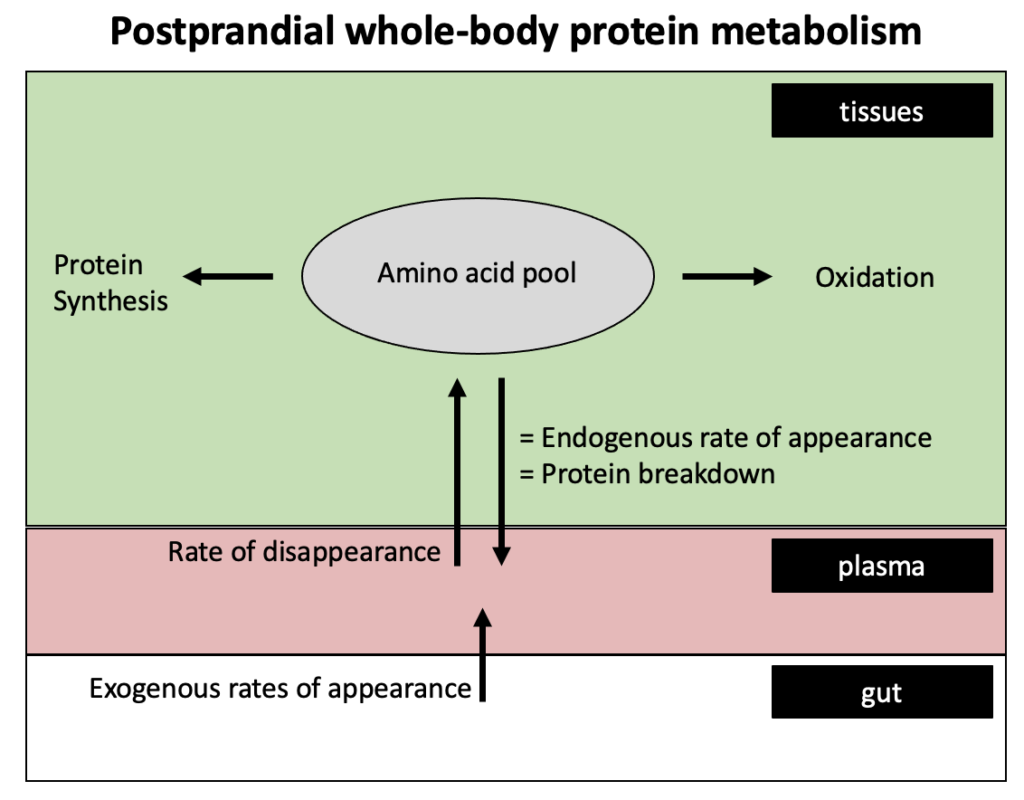
All living tissues are constantly renewed in a process called protein turnover, which allows a high tissue quality to be maintained. For example, muscle tissue has a protein turnover rate of 1-2 % per day, which translates to muscle tissue being completely renewed every 2-3 months.
Protein turnover is regulated by two opposing processes: protein breakdown in which body proteins are broken down to amino acids, and protein synthesis in which amino acids are incorporated into body proteins.
An imbalance between protein synthesis and breakdown results in a net gain (synthesis > breakdown) or net loss (breakdown > synthesis) in body protein.
It is well-established that protein ingestion and exercise can stimulate muscle protein synthesis.
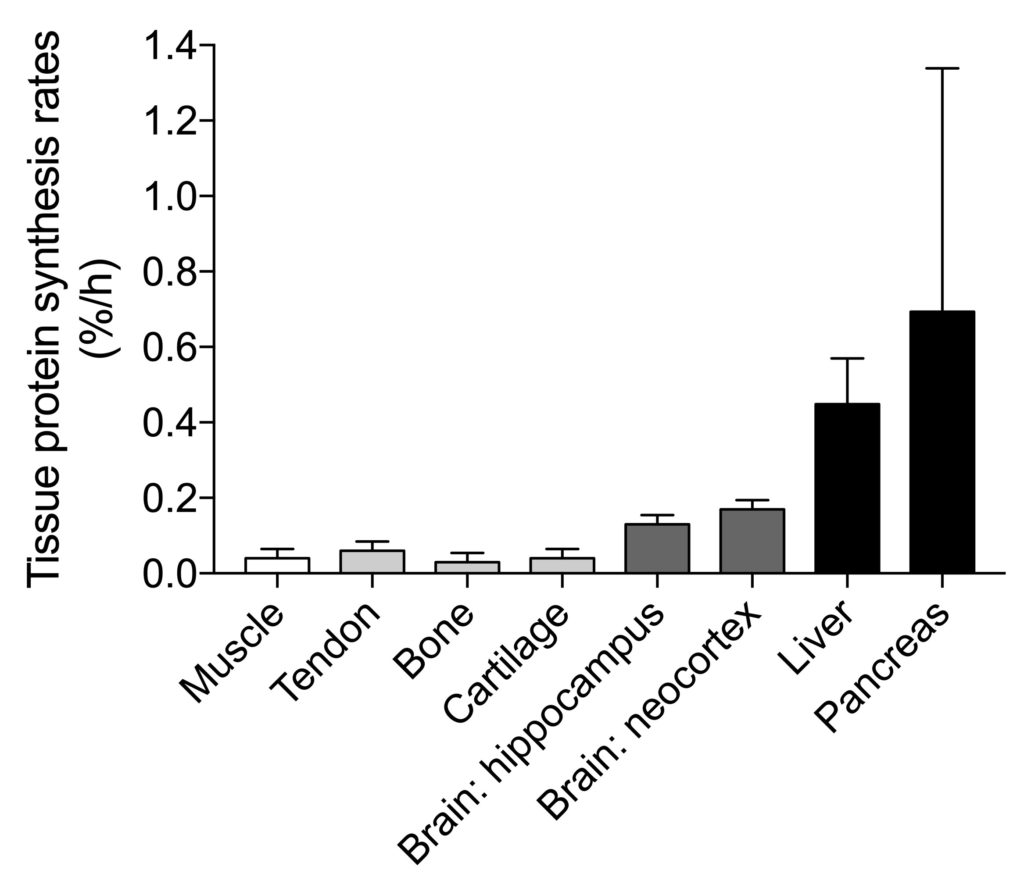
However, several organs such as the brain, liver, and pancreas, show substantially higher (basal) protein synthesis rates when compared to skeletal muscle tissue.
It remains to be determined whether protein synthesis rates in these tissues can be modulated by external stimuli such as protein ingestion. It likely can, but this is difficult to establish as taking a biopsy from these tissues is too invasive.
Whole-body protein synthesis rates are highly responsive to protein intake. As the contribution of muscle protein synthesis rates to whole-body protein synthesis rates is relatively, small despite the large amount of muscle mass, this suggests that other organ tissues may also be responsive to (protein) feeding
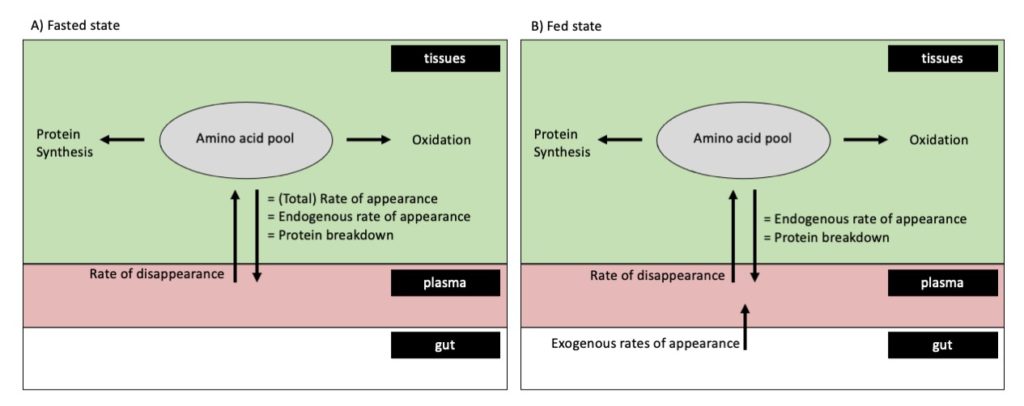
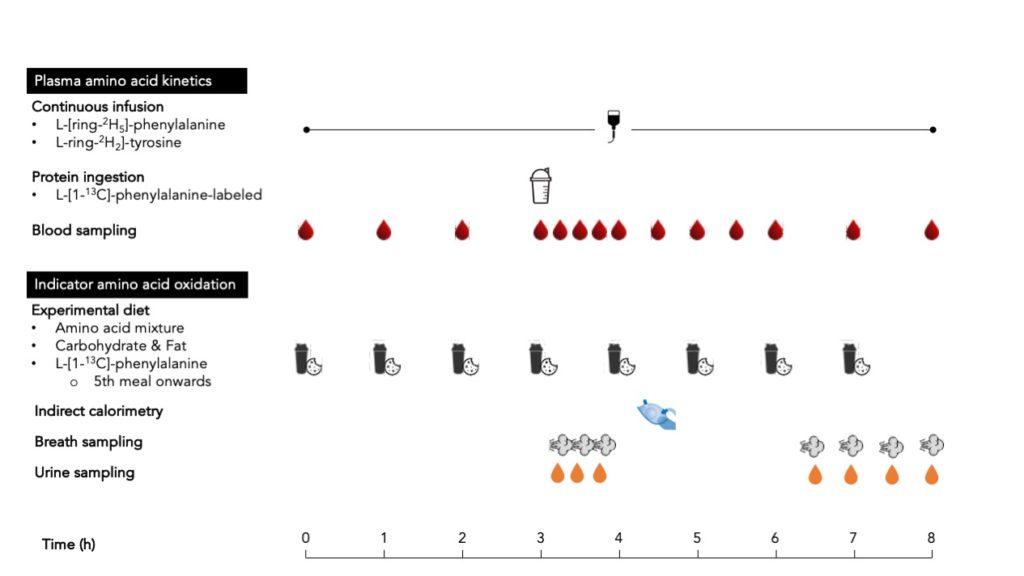
Whole-body protein synthesis rates in the fasted or fed state can be quantified by measuring plasma amino acid kinetics, although the latter requires the production of intrinsically labeled protein.
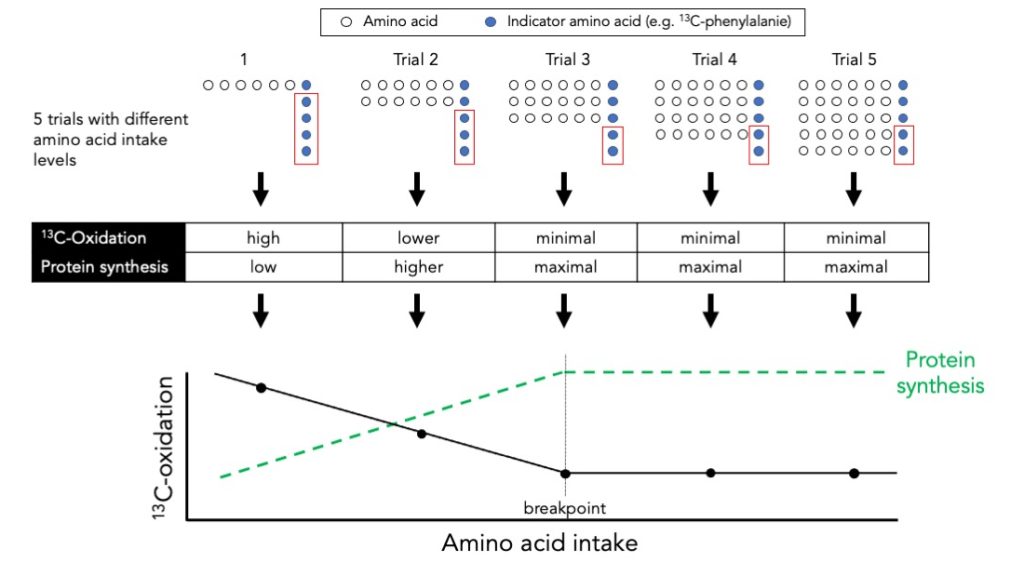
Protein intake requirements to maximize whole-body protein synthesis may also be determined by the indicator amino acid oxidation (IAAO) technique.
Advantages of the intrinsically labeled protein method are that it allows for whole-body protein synthesis, breakdown, and net balance rates to be assessed and that it can be used for bolus feeding which is representative of a normal meal pattern.
The only real drawback of the intrinsically labeled protein method is that it is very expensive.
The main advantages of the IAAO technique are that is minimally invasive and well suited to perform multiple tests on the same subject.
The disadvantages of the IAAO technique are that it uses free amino acids instead of actual protein, it uses a sip feeding protocol, and that it can only determine the protein requirements to maximize protein synthesis.
The IAAO technique has also been used to calculate whole-body protein synthesis, breakdown, and net balance. However, this is based on incorrect calculations which I explain in the review.
Most papers claiming to measure whole-body protein metabolism use poor methodology (this includes the IAAO method).
Even with the best method (intrinsically labeled protein), it is unclear how useful whole-body protein metabolism is for practical inferences.
For example, it’s clear that a positive protein balance in muscle allows the muscle to grow and adapt to training. However, it’s unclear if other organs also grow in response to a positive whole-body protein balance and whether that has functional benefits or potential negative effects.
So for practical recommendations, muscle protein synthesis is a more useful outcome as compared to whole-body protein balance.
The full paper goes in way more details. Please let me know if you have comments and/or question!
Our OPEN-ACCESS paper:
Trommelen et al. Assessing the whole-body protein synthetic response to feeding in vivo in human subjects. Proc Nutr Society, 2021

Thanks for this content