Muscle protein synthesis is the process of building muscle mass.
Muscle protein synthesis is essential for exercise recovery and adaptation. As such, it’s a really popular topic in the fitness community.
But the methods used to measure muscle protein synthesis in studies are very complicated. Some basic knowledge of the various methods is essential to draw proper conclusions from the research you’ll read.
The purpose of this article is to provide a comprehensive guide on muscle protein synthesis: what is it, how is it measured, what are the strengths and limitations, how to draw proper conclusions from muscle protein synthesis research, and of course practical guidelines how to optimize it.
I’ve made a table of contents, so you can jump to specific sections of interest or reference a specific section.
Please note that section 3 described the various methods to measure muscle protein synthesis. You might want to skip this section if you just want exercise and nutrition guidelines to optimize gains. Perhaps you can come back to it later when you are ready to become a true muscle protein synthesis research master.
I’ll be covering:
Contents
- 1. What is protein synthesis?
- 2. Why muscle protein breakdown is of less importance
- 3. Methods to measure protein synthesis
- 4. Interpretation and misconceptions
- 5. Advantages of muscle protein synthesis measurements over muscle mass measurements.
- 6. How to optimize muscle protein synthesis: exercise guidelines.
- 7. How to optimize muscle protein synthesis: nutrition guidelines
- 8. Summary
1. What is protein synthesis?
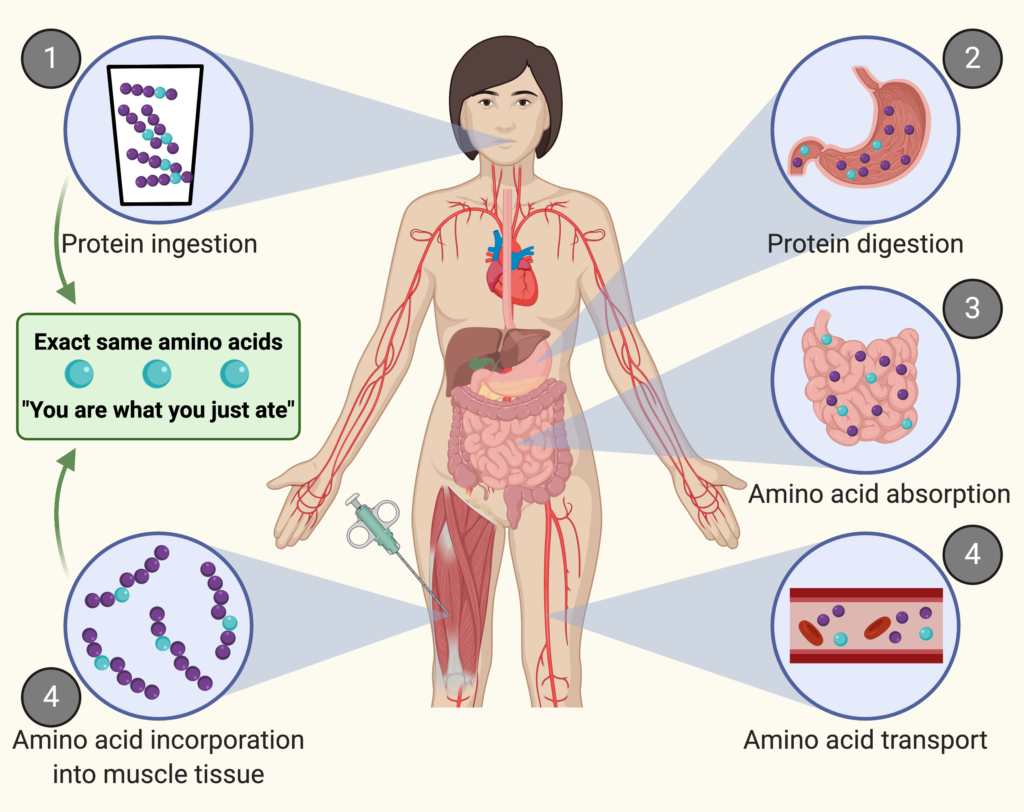
Protein is the main building block of your muscle.
When you ingest protein, the protein is digested into amino acids. These amino acids are absorbed in the gut and subsequently released into the circulation. From there, the amino acids are transported to peripheral tissues where they are taken up and can be build into tissue protein.
Protein synthesis is the process of building new proteins. This process happens in all organs. Muscle protein synthesis is the process of building specifically muscle protein.
Think of a muscle as a wall. Each brick is an amino acid. Muscle protein synthesis is the addition of new bricks to the wall.
Now, this would mean the wall would become larger and larger. However, there is an opposing process. On the other side of the wall, a process called muscle protein breakdown is removing bricks. Muscle protein breakdown is also commonly referred to as muscle proteolysis or muscle degradation.
It’s important to realize that both muscle protein synthesis and breakdown are always ongoing. They are not “on” or “off”, but their speed can increase or decrease. The difference in speed of these two opposing processes determines the net change in muscle protein size.
If muscle protein in synthesis exceeds muscle protein breakdown, the wall will become larger (your muscles are growing). If muscle protein breakdown exceeds muscle protein synthesis, the wall is shrinking (you’re losing muscle mass).
The sum of these two processes determines your net balance:
net muscle protein balance = muscle protein synthesis – muscle protein breakdown.
You can also compare it to your bank account.
balance = income – expenses
2. Why muscle protein breakdown is of less importance
We’ll almost exclusively talk about muscle protein synthesis, and not focus much on muscle protein breakdown.
That might sound like you’re missing out on a whole lot, but you’re not.
Changes in muscle protein synthesis are much greater in response to exercise and feeding than changes in muscle protein breakdown in healthy humans (Phillips, 1997)(Greenhaff, 2008).
While feeding can reduce muscle protein breakdown by approximately 50%, very little is needed to reach this maximal inhibition.
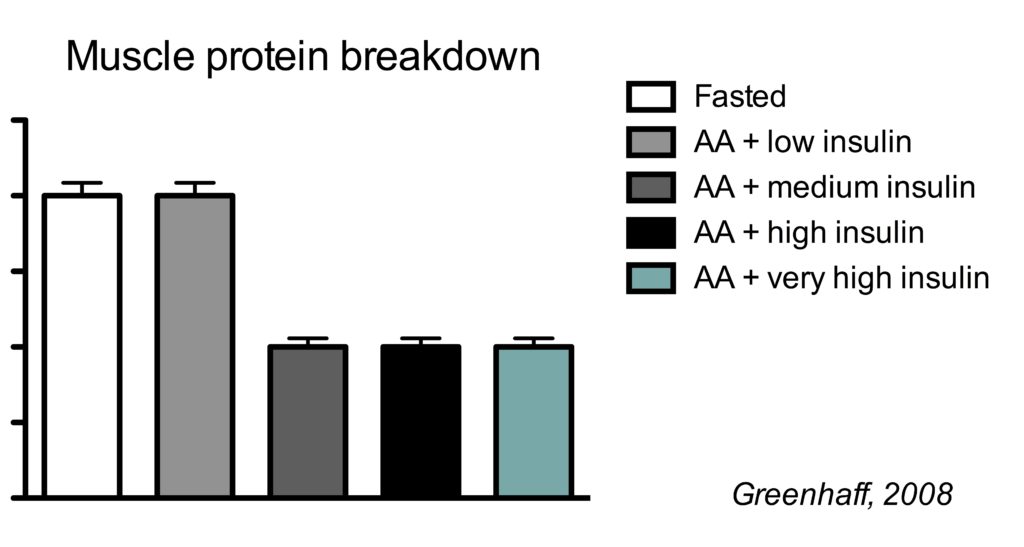
This is best illustrated by a study which clamped (maintained) insulin at different concentrations and also clamped amino acids at a high concentration.
There were five conditions:
- Fasted state
- high amino acids + low insulin
- high amino acids + medium insulin
- high amino acids + high high insulin
- high amino acids + very high insulin
In the illustration below, you see the effects on muscle protein breakdown.
In a fasted state, muscle protein breakdown rates were relatively high (condition 1). Amino acid infusion did not reduce muscle protein breakdown when insulin was kept low (condition 2). But when insulin was infused to reach a moderate concentration, muscle protein breakdown rates went down (condition 3). Further increasing insulin did not have an additional effect on muscle protein breakdown (conditions 4 and 5).
So this study shows us a couple of things.
Firstly, insulin inhibits muscle protein breakdown, but you only need a moderate insulin concentration to reach the maximal effect. In this study the medium insulin concentration already resulted in the maximal 50% muscle protein breakdown, but other research has shown that even half the insulin concentration of the medium group is already enough for the maximal effect (Wilkes, 2009).
Secondly, protein ingestion does not directly inhibit muscle protein breakdown. While protein intake can decrease muscle protein breakdown (Groen, 2015), this is because it increases the insulin concentration.
You only need a minimal amount of food to reach insulin concentrations that maximally inhibit muscle protein breakdown. In agreement, adding carbohydrates to 30 g of protein does not further decrease muscle protein breakdown rates (Staples, 2011).
Therefore, it often doesn’t make much sense to measure muscle protein breakdown in nutrition studies. All study groups that got at least some amount of food will have the 50% inhibition of fasted muscle protein breakdown rates. If the effect on muscle protein breakdown is the same between groups, then changes in muscle protein net balance would be entirely be explained by differences in muscle protein synthesis.
It should be noted that HMB decreases muscle protein breakdown in an insulin independent way (Wilkinson, 2013). It is not known of the effects of HMB and insulin on muscle protein breakdown are synergistic. However, HMB supplementation appears to have minimal effects of muscle mass gains in long-term studies (Rowlands, 2009).
Of course, you can speculate that muscle protein breakdown becomes more relevant during catabolic conditions during which there is significant muscle loss, such as dieting or muscle disuse (e.g. bed rest or immobilization). However, it cannot be simply assumed that the observed muscle loss in such condition is the result of increased muscle protein breakdown. After just 3 days of dieting, there is already a large decrease in muscle protein synthesis. In agreement, there is a large decrease in muscle protein synthesis during muscle disuse. Therefore, muscle loss may be largely (or even entirely) caused by a reduction in muscle protein synthesis, and not by an increase in muscle protein breakdown.
But let’s say that muscle protein breakdown does go up a bit during catabolic conditions. Then the first question would be, could nutrition prevent this? If the answer is no, muscle protein breakdown is still not that relevant to measure. If nutrition does have an effect, how much would it take? Let’s say you need double the amount of insulin you normally need to maximally inhibit muscle protein breakdown. That would still be a small amount of insulin that any small meal would release and all nutritional interventions have the same effect. So even during catabolic conditions, muscle protein synthesis rates are likely much more relevant than muscle protein breakdown.
While it sounds that muscle protein breakdown is a bad thing and we should try to completely prevent it, that is not necessarily true. Muscle proteins get damaged from exercise, physical activity, and metabolism (e.g. oxidative stress, inflammation etc). Muscle protein breakdown allows you to break down those damaged muscle proteins into amino acids and recycle most of them into new functional muscle proteins again.
In fact, muscle protein breakdown has beneficial roles in muscle growth and adaptation! If mice are genetically engineered so that they can’t properly break down muscle protein, they are actually weaker and smaller than normal mouses. This highlights that at least some amount of muscle protein breakdown is necessary to optimally adapt to training and maximize muscle growth (Bell, 2016).
So anytime you eat just about anything, you’ll reduce muscle protein breakdown by 50%. We don’t know how to reduce muscle protein breakdown even further, but it’s not clear if we would even want that, as at least some muscle protein breakdown seems necessary for optimal muscle growth.
3. Methods to measure protein synthesis
3.1 Nitrogen balance
Carbohydrates and fats are made of carbon, hydrogen, and oxygen. In contrast, protein also contains nitrogen. So the nitrogen that we get through our diet has to come from protein.
As protein is broken down by the body, most of the protein derived nitrogen has to be excreted in the urine or it would accumulate and become toxic.
It’s fairly easy to measure nitrogen in food, feces, and urine. By doing so, we can calculate a balance:
nitrogen balance = nitrogen intake – nitrogen excretion
If nitrogen intake is bigger than nitrogen excretion, we are in a positive nitrogen balance. This indicates that your body stores more protein than it’s losing. This gives a general view that the body is in an anabolic (growing) state.
However, this method gives us very little insight into what exactly is going on.
You can have a positive nitrogen balance, while you’re losing muscle tissue. For example, your body might be building gut protein at a rate that exceeds your muscle loss.
As such, nitrogen balance is not that informative for athletes.
Example paper nitrogen balance: (Freeman, 1975)
3.2 Tracers
Before we go on to the next method, I need to introduce a new concept: tracers.
Tracers are compounds that you can trace throughout the body. Amino acid tracers are the most common type of tracers to assess muscle protein synthesis. These are amino acids that have an additional neutron. These amino acid tracers function identically to normal amino acids. However, they weigh slightly more than a normal amino acid, which allows us to distinguish them from normal amino acids.
A normal carbon atom has a molecular weight of 12. When we add a neutron, it has a weight of 13. We indicate these special carbons like this: L-[1-13C]-leucine. This means the amino acid leucine, has a carbon atom with a weight of 13. When you see such a notation in a paper, you know they’re using tracers.
Because you can follow amino acid tracers throughout the body, it allows us to measure various metabolic processes that happen to the amino acids, including protein synthesis.
3.3 Whole-body protein metabolism
Using amino acid tracers, we can measure protein synthesis, breakdown, oxidation and net balance.
Note that protein synthesis refers to protein synthesis of any protein in the body (whole-body protein synthesis). Again, do not mistake it for muscle protein synthesis, which is protein synthesis specifically of muscle protein. Therefore, (whole-body) protein synthesis measurements are not necessarily relevant for athletes and might actually give you the wrong impression (as will be discussed in chapter 3).
However, whole-body protein metabolism data provides more insight than the nitrogen balance method.
Nitrogen balance only indicates an overall anabolic or catabolic state. The whole-body protein metabolism method also indicated this by a positive or negative protein net balance. However, it also shows whether changes in net balance are because of an increase in protein synthesis, a decrease in protein breakdown, or a combination of both.
Please note that this does not contradict the earlier discussion on muscle protein breakdown is not that important. While muscle protein breakdown doesn’t change much, protein breakdown of other tissues can change drastically.
Instinctually, you might think that a more positive whole-body protein balance must be a good thing. However, that’s not really the case. It could simply mean that you’re building more gut protein, which is not something you would necessary want unless you want to have a bloated stomach.
Example paper whole-body protein metabolism: (Borie, 1997)
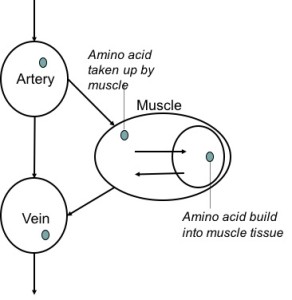
3.4 Two pool and three pool arteriovenous technique
These methods measure amino acid concentrations in the artery to a muscle, and the vein from that muscle.
Let’s say that the amino acid concentration is low in the artery going to a muscle and high in the vein coming from that muscle. Where did those extra amino acids in the vein come from? They’re released from the muscle, and it would indicate that the muscle is breaking down. Conversely, if the muscle would take up a lot of amino acids from the artery, but releases less amino acids to the vein, it would indicate that the muscle is taking up a lot of amino acids to build muscle proteins.
This method is called the two pool arteriovenous method. A drawback of this method is that you don’t really know for sure what is going on in the muscle. Just because a muscle is taking up amino acids, that does not mean it’s building all of those amino acids into actual muscle tissue.
To solve this problem, this method can be combined with muscle biopsies. This is called the three pool model and allows us to see what happens to the amino acids once they’re taken up by the muscle.
The main advantage of this method is that it measures both muscle protein synthesis and muscle protein breakdown. However, the calculations are depended on blood flow measurements, which we can’t measure all that accurately. Therefore, this is not the preferred method for measuring muscle protein synthesis.
Example paper 3 pool model: (Rasmussen, 2000)
3.5 Fractional synthetic rate
This method combines the infusion of amino acid tracers with muscle biopsies.
The most basic explanation is that you take a pre and post muscle biopsy, and measure the rate at which the amino acid tracer is built into the muscle.
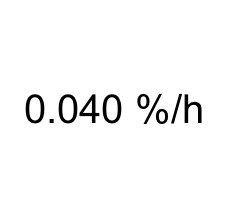
This method gives you a fractional synthetic rate (FSR), expressed as %/h. It shows you how fast a muscle would rebuild itself entirely. An FSR of 0.04 %/h means that each hour, 0.04% of the total muscle is synthesized. This translates to a completely new muscle every 3 months. To regenerate as fast as Wolverine of the X-men, you would need an FSR of 100000 %/h in all your tissues.
This is the most common method of measuring muscle protein synthesis.
A minor drawback of this method is that it requires a ”steady state” to have the most accurate measurements. I don’t want to go into too many details, but it often means plasma amino acid enrichment should stay at the same level (the ratio of the tracer amino acid to the normal amino acid). However, protein ingestion would disturb this steady state, as a lot of normal amino acids will enter the blood, thus throwing off the tracer amino acid to normal amino acid ratio. Therefore, a lot of older studies provided protein in small sips, which wouldn’t disturb the steady state.
A slight disturbed steady state isn’t the end of the world for the measurement, but it’s sometimes something to consider when evaluating the data.
However, our lab has gotten fancy in this area. We have been able to produce highly enriched intrinsically labeled protein. This means that the amino acid tracers have been build into our protein supplements. So as our intrinsically protein supplements are absorbed, both amino acid tracers and normal amino acids enter the blood. Therefore the steady state is not disrupted and FSR can be calculated more accurately.
Example paper FSR (with and without intrinsically labeled protein): (Holwerda, 2016)
3.6 De novo muscle protein synthesis
The highly enriched intrinsically labeled protein has another advantage.
You can trace the amino acids from the protein: first, as they are digested, then as they appear in the blood, subsequently they are taken up by the muscle, and ultimately some of them are built into actual muscle tissue. So we can measure how much of the protein you eat, actually ends up into muscle tissue.
This is called de novo muscle protein synthesis. It’s building new muscle protein from your food, in contrast to building muscle protein by recycling amino acids from protein breakdown.
Example paper de novo muscle protein synthesis: (Trommelen, 2017).
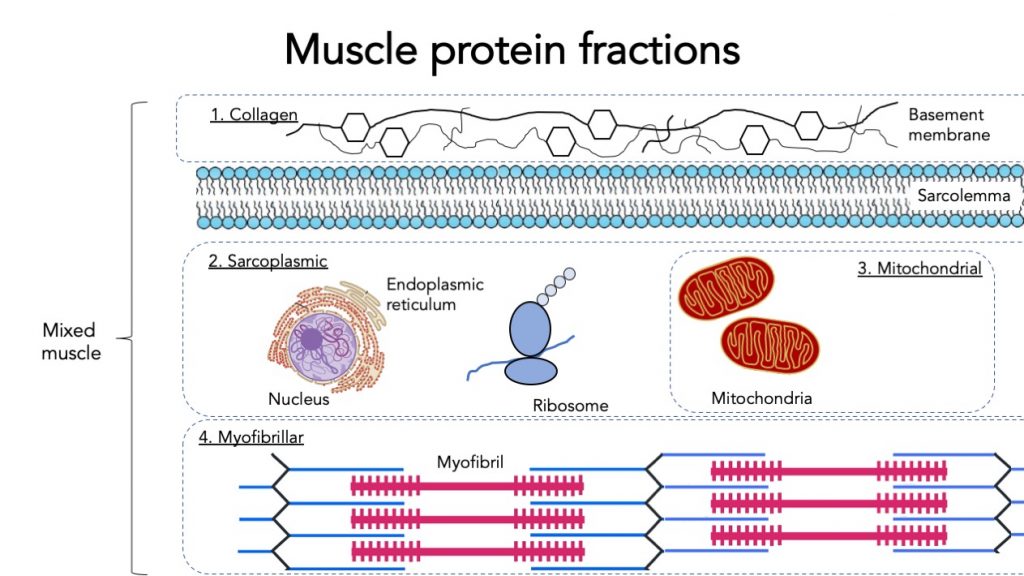
3.7 Specific muscle protein fractions
When measuring muscle protein synthesis, we can measure mixed muscle protein synthesis (all types of muscle protein together). But muscle protein can be further specified into fractions.
The main muscle protein fraction is myofibrillar protein. Myofibrillar proteins contract and represent the majority of the muscle mass. This fraction is highly relevant for building muscle mass and strength.
Mitochondrial proteins only represent a small part of the muscle. Mitochondria are the powerhouses of the muscle, they burn carbohydrate and fat for fuel. Therefore mitochondrial protein synthesis is more informative about energy production capacity in the muscle and more relevant for endurance athletes and metabolic health.
Sarcoplasmic protein contains various organelles such as the endoplasmic reticulum and ribosomes.
Intramuscular connective tissue protein represents collagen protein in the muscle. This collagen helps transfer the muscle force generated by myofibrillar protein. It may be relevant for strength, injury prevention, and mobility, but we don’t know that much about it yet.
Example paper myofibrillar vs mitochondrial protein synthesis: (Wilkinson, 2000), or intramuscular connective tissue protein synthesis (Trommelen, 2020)
- Mixed muscle protein synthesis measures the synthesis of all muscle proteins.
- Myofibrillar protein synthesis measures only contractile proteins and is the most relevant measurement for muscle mass gains.
- Mitochondrial protein synthesis is more relevant for endurance and metabolic health.
3.8 Deuterium oxide
More recently, deuterium oxide (D2O, also called heavy water) is getting popular to measure muscle protein synthesis.
I’m going to skip most of the technical details, but the big difference with amino acid tracers is that this technique can accurately measure muscle protein synthesis days or even weeks, whereas the amino acid tracer technique measures muscle protein synthesis accurately over hours. In the next section, we’ll explain why this matters.
Example paper deuterium oxide: (Brooks, 2015)
3.9 Molecular markers
There is evidence that a variety of signaling molecules are involved in the regulation of muscle protein synthesis. Most notably, the protein from the mTOR pathway. Research of these molecular markers is very important to better understand how physiological processes are regulated and ultimately can be influenced by exercise, nutrition or even drugs.
However, at this point, we don’t understand them well for them to be useful predictors of physiology. In other words, while mTOR activation is involved in the regulation of muscle protein synthesis, an increase in mTOR activation doesn’t necessarily mean that muscle protein synthesis will actually increase.
Therefore, you should be very skeptical to draw conclusions based on studies that only measure molecular markers of muscle protein synthesis and muscle protein breakdown.
Example paper molecular markers (in this paper they don’t reflect actual MPS measurements): (Greenhaff, 2008)
4. Interpretation and misconceptions
Methods are not necessarily good or bad. But the interpretation of the data based on these methods can be wrong.
4.1 Whole-body protein synthesis vs muscle protein synthesis
We recently showed that resistance exercise does not increase whole-body protein synthesis (Holwerda, 2016). So should we conclude that resistance exercise is not effective to build muscle?
Absolutely not.
Whole-body protein metabolism measures the synthesis of all proteins in the body. Resistance exercise specifically builds muscle protein; it doesn’t increase protein synthesis in other organs. Other tissues in the body have much higher synthesis rates, and therefore, the muscle only has a relatively small contribution to the total whole-body protein synthesis rates. Therefore, you don’t see an increase in whole-body protein synthesis following resistance exercise.
In the same study, we also measured muscle protein synthesis (using the FSR method both with and without intrinsically labeled protein) and de novo muscle protein synthesis. All 3 methods showed that resistance exercise was anabolic for the muscle.
So resistance exercise did exactly what it’s supposed to do: build muscle, not build your other organs.
Often, labs don’t have the money or expertise to do many of these measurements. Imagine we would have only measured whole-body protein synthesis. Our study would give the wrong impression that resistance exercise is not anabolic, as we saw no increase in whole-body protein synthesis rates. Therefore it’s important that you understand the difference between whole-body protein synthesis and muscle protein synthesis methods.
Let’s discuss another example:
This study has been getting a lot of attention:
”The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults” (Kim, 2016).
Shortly, it says that very large protein meals are beneficial because they reduce protein breakdown.
This gathered a lot of attention on social media and some of the comments were:
– See we shouldn’t ignore muscle protein breakdown!
– See that you need more than 40 gram of protein in each meal!
Again, this study gave the wrong impression, because whole-body protein breakdown was mistaken for muscle protein breakdown (the latter was not measured in this study). In case you’re wondering, this study did measure muscle protein synthesis. Both the 40 gram and the 70 gram dose were equally effective at stimulating muscle protein synthesis. So this study doesn’t indicate that you need more than 40 gram of protein each meal as an athlete.
4.2 Correlation muscle protein synthesis and muscle mass gains
One of the purposes of measuring muscle protein synthesis is to study if an intervention helps to build muscle or maintain muscle mass.
On social media, I occasionally see people dismissing muscle protein synthesis studies by claiming:
”it’s just an acute mechanistic study, it doesn’t necessarily translate to long-term changes in muscle mass”.
While there is some truth to the statement, it’s massively overused and often used as a cop-out as they have limited understanding of muscle protein synthesis. In fact, there are strong benefits for acute muscle protein synthesis studies over long-term training studies, which I’ll discuss in the next section.
Let me first get something out of the way: we have no bias for either acute or long-term studies. We run both at our lab, and do some of the most expensive studies in the field of either type.
The concern that muscle protein synthesis may not translate to muscle mass gains got an uprise when the following study was published:
”Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men” (Mitchell, 2014).
A lot of people seem to think that based on this study, muscle protein synthesis measurements do not translate to actual muscle mass gains in the long term.
But that conclusion is way beyond the context of the study. This study measured muscle protein synthesis in the 6 hours after a single exercise bout. However, resistance exercise can increase muscle protein synthesis for several days. So a 6-hour measurement does not capture the entire exercise response. This study showed that measuring muscle protein synthesis for 6 hours does not predict muscle mass gains. That is totally different from the conclusion that muscle protein synthesis (regardless of measurement time) does not predict muscle mass gains.
This was followed up by a study which used the deuterium oxide method to measure muscle protein synthesis rates during all the weeks of training (not just a few hours after one session), and found that muscle protein synthesis did correlate with muscle mass gains during the training program (Brooks, 2015).
More recently, a study found that muscle protein synthesis measured over 48 hours after an exercise bout did not correlate with muscle mass gains in untrained subjects at the beginning of an exercise training program, but it did at three weeks of training and onwards (Damas, 2016). While untrained subjects have a large increase in muscle protein synthesis after their initial exercise sessions, they also have a lot of muscle damage. So muscle protein synthesis is mainly used to repair damaged muscle protein, not to grow. After just 3 weeks of training, muscle damage is diminished, and the increase in muscle protein synthesis is actually used to hypertrophy muscles.
So do these studies show that muscle protein synthesis predicts muscle mass gains, but only in the right context.
- Exercise elevates muscle protein synthesis for 24 hour or longer: ideally you have a muscle protein measurement of 24 hours or longer and have ‘trained’ subjects so training induced muscle damage is attenuated (3 weeks of training is enough)
- Protein ingestion increased muscle protein synthesis for ±3-6 hours, so ideally you have measurement period of 6 hours (longer would actually be detrimental)
5. Advantages of muscle protein synthesis measurements over muscle mass measurements.
A huge benefit of muscle protein synthesis studies is that they are more sensitive than studies that measure actual muscle mass gains. This means that muscle protein synthesis studies can detect an anabolic effect easier than long term studies which simply miss it (long term studies might draw the wrong conclusion that something does not benefit muscle growth when it actually does).
For example, it has been shown time and time again that protein ingestion increases muscle protein synthesis. But the vast majority of long-term protein supplementation studies (80%) have found that protein supplementation does not increase muscle mass gains (Cermak, 2012).
This is because most long term studies are underpowered, aka don’t have enough statistical power to find small positive effects. Muscle mass gain is simply a very slow process. You need to do a huge study, with a huge amount of subjects, who consume additional protein for many months, before you will actually see a measurable effect of protein supplementation.
We performed a meta-analysis (combining the results of individual studies) on the effect of protein supplementation on muscle mass gains. We demonstrated that only 5 studies concluded that protein supplementation had a benefit, while 17 did not! However, most of the studies that showed no significant benefit, did show a small (non-significant) benefit. When you combine all those results, you increase the statistical power and you can conclude that protein supplementation actually does improve muscle mass. So in this case, most long-term studies gave the wrong impression, and muscle protein synthesis studies are actually preferred.
There are a lot of long-term studies that have a relative small number of subjects and a small study duration and conclude that an intervention did not work (for example, protein supplementation, or X versus Y set of exercise for example). However, the studies were doomed to begin with. They needed to be 3 times as big and 2 times as long to have a chance to find a positive effect. These studies unfortunately give people the impression that something doesn’t work, while it actually might/does.
Now if the effect of giving additional protein is already extremely hard to detect in long-term studies, how realistic is it to find smaller effects? For example, optimizing protein intake distribution throughout the day has been shown to optimize muscle protein synthesis rates (Mamerow, 2014)(Areta, 2013). However, this effect is smaller than adding another protein meal. So the effect of protein distribution is almost impossible to find in a long-term study. For such a research question, acute muscle protein synthesis studies are simply much better suited.
The second big benefit of muscle protein synthesis studies is that they give a lot more mechanistic insight. They help you understand WHY a certain protein is good or not that good at stimulating muscle protein synthesis (for example, its digestion properties, amino acid composition etc). These kinds of insights help to better understand what triggers muscle growth and come up with new research questions. These kind of insights are very hard to obtain in long-term studies, which typically only show the end result of the mechanisms.
The benefits of measuring muscle protein synthesis include the sensitivity, controlled environment, and they allow you to investigate questions that are almost impossible to answer in long-term studies. In addition, they’ll give you a lot of mechanistic insight, which opens the door for future research.
But ultimately, it’s not a matter of which type of study is better. Again, we do both and each has its purpose and build on each other. Usually, muscle protein synthesis studies are performed to see if something work (as they are very sensitive) and why it works. Once we think we have a good understanding, then we’ll try to see if the concept has the expected effect in a long-term study. Only when you have both, you have pretty convincing evidence that your intervention does what you claim it to do.
6. How to optimize muscle protein synthesis: exercise guidelines.
Now it’s time to translate the muscle protein synthesis to some practical recommendations.
6. 1 Number of sets
Multiple sets increase muscle protein synthesis more than a single set (Burd, 2010). A higher weekly training volume (number of sets to muscle) results in a greater muscle mass gains (Schoenfeld, 2016).
6.2 Reps per set
It is often recommended that a rep range of 8-12 reps per set is optimal for muscle growth. The American College of Sport Medicine position stand states (ACSM, 2009):
For novice (untrained individuals with no RT experience or who have not trained for several years) training, it is recommended that loads correspond to a repetition range of an 8-12 repetition maximum (RM). For intermediate (individuals with approximately 6 months of consistent RT experience) to advanced (individuals with years of RT experience) training, it is recommended that individuals use a wider loading range from 1 to 12 RM in a periodized fashion with eventual emphasis on heavy loading (1-6 RM) using 3- to 5-min rest periods between sets
However, these recommendations lack evidence. More recently, Stu Phillip’s lab has performed a series of studies demonstrating that repetition load plays a minimal, if not negligible, role in the hypertrophic response to resistance exercise, if sets at the different rep ranges are performed to failure (Burd, 2010)(Morton, 2016).
The main takeaway here is that there are no magic rep ranges that are superior for muscle growth.
6.3 Training to failure
It is unclear whether each set should be taken to failure. Muscular failure decreases performance on subsequent sets, thereby reducing training volume. In addition, it has been suggested that frequent, high volume training with each set to taken to failure results in ‘overtraining’, eventually forcing reduced training volume or intensity for recovery (Stone, 1996).
Perhaps performing a set with 1-2 reps left in the tank will still give a near-maximal stimulus to the muscle, without much of the associated fatigue. If sets are not taken close to failure, the muscle protein synthetic response will be small (Burd, 2010). But at least in untrained subjects, training close to failure appears to produce similar muscle mass gains as training to complete failure (Nóbrega, 2017).
6.4 Rest between sets
A longer rest period between sets increases the larger post-exercise muscle protein synthetic response compared to a short rest period (5 vs 1 min)(McKendry, 2016). In agreement, a longer interset rest period improves muscle mass gains compared to a shorter rest period (3 vs 1 min) (Schoenfeld, 2016).
6.5 Training frequency
A single bout of resistance exercise can stimulate muscle protein synthesis for longer than 72 hour, but peaks at 24 h (Miller, 2005). This suggests that the popular so called ‘bro-split’ (hitting each muscle group once a week on different training days) is suboptimal. Indeed, training each muscle group at least twice a week results in larger muscle mass gains (Schoenfeld, 2016).
6.6 Training status
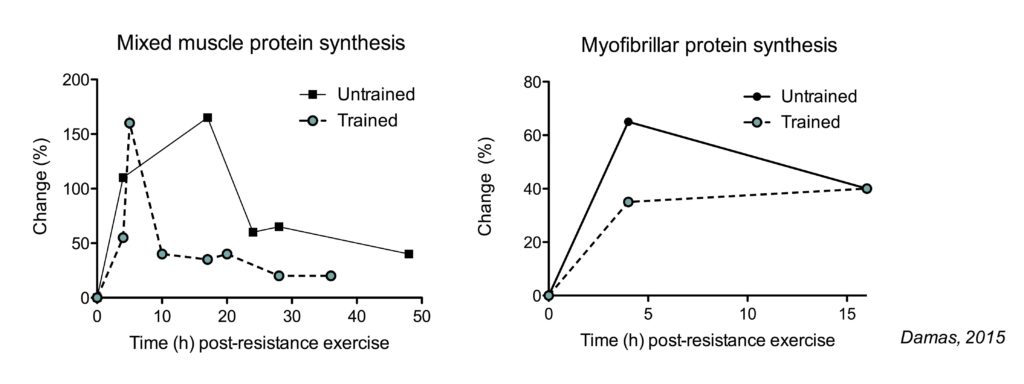
The total muscle protein synthetic (MPS) response (determined by the increase in MPS rates and the duration of these increased rates) is decreased in trained subjects compared to untrained subjects (Damas, 2015). However, the pattern of this decreased response is differs between mixed muscle protein synthesis (the synthesis of all types of muscle proteins) and myofibrillar protein synthesis (the synthesis of contractile proteins: the relevant measurement for muscle mass).
The increase in mixed muscle protein synthesis is shorter lived in trained subjects. In contrast, myofibrillar protein synthesis rates do not increase as much in trained subjects, but the duration of the increase does not appear impacted.
The larger increase in the total muscle protein synthetic response seems like a logical explanation why untrained people can make faster much gains than experienced lifters. However, this is not necessarily true.
In untrained subjects, there is not only a large increase myofibrillar protein synthesis, but also in muscle damage following resistance exercise. A large portion of the myofibrillar protein synthesis is used to simply repair damaged muscle proteins, rather than growing muscle proteins. In more trained subjects, here is a smaller increase in myofibrillar protein synthesis, but there is also much less or even minimal muscle damage following resistance exercise (just 3-10 weeks of training is enough to see these effects). This means that in a trained state, the increase in myofibrillar protein synthesis can actually be used to actually increase muscle mass. When you correct for muscle damage, myofibrillar protein synthesis rates measured over 48 hour post-exercise recovery are similair in untrained subjects and after 10 weeks of training (Damas, 2016).
Of course, most athletes would hardly consider someone trained after just 10 weeks. Unfortunately, little is know about how years of serious training impacts the muscle protein synthetic response to resistance exercise. While it’s tempting to speculate on how and if advanced athletes should modify training variables compared to untrained subjects, there is no solid evidence to support such suggestions.
7. How to optimize muscle protein synthesis: nutrition guidelines
7.1 Amount of protein
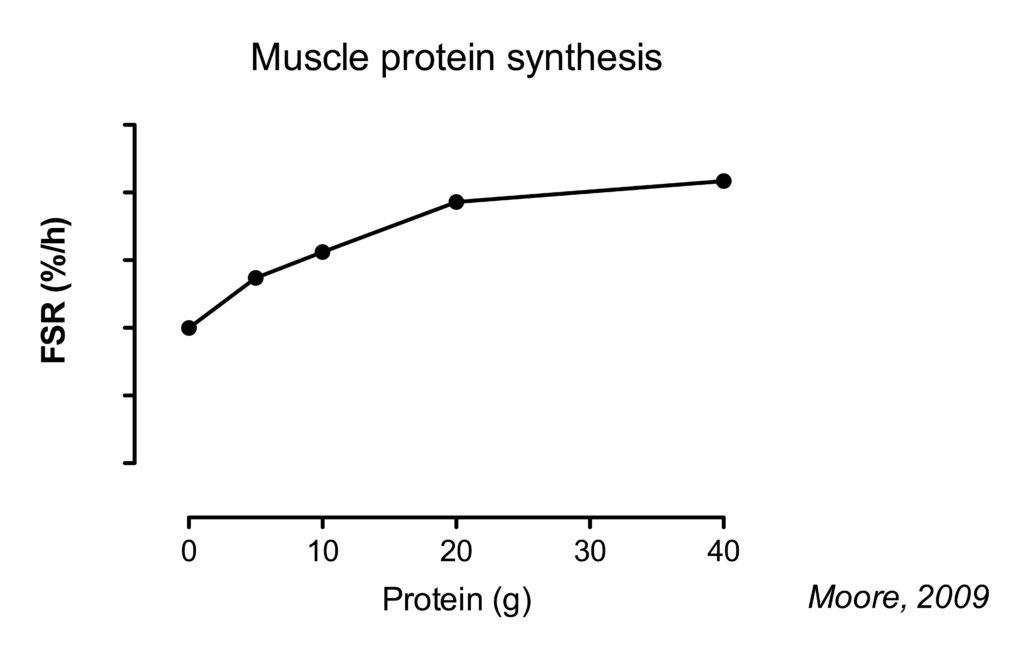
Twenty gram of protein gives a near-maximal increase in MPS after lower body resistance . Going up further to 40 g, results in a approximately 10% higher MPS rates (Moore, 2009)(Witard, 2014).
When data of several studies was combined and the amount of protein was expressed per bodyweight, it was found that on average 0.24 g/kg bodymass (or 0.25 g/kg lean body mass) would optimize muscle protein synthesis. However, the authors suggest a safety margin of 2 standard deviations to account for inter-intervidual variability, resulting in a dose of protein that would optimally stimulate MPS at an intake of 0.40 g/kg/meal (Moore, 2015).
More recently, it has been shown that the amount of lean body mass does not impact the response to protein ingestion (Macnaughton, 2016). In other words: bigger dudes don’t need more protein to get the same response as smaller guys.
However, this study found that 40 g of protein resulted in approximately 20% higher higher MPS rates compared to 20 g. The authors speculated that this was related to the fact that this was following a session of whole-body resistance exercise compared to the lower-body exercise used in previous studies.
7.2 Protein source
Protein sources differ in their capacity to stimulate MPS. The main properties that determine the anabolic effect of protein are it’s digestion rate and it’s amino acid composition (particularly leucine).
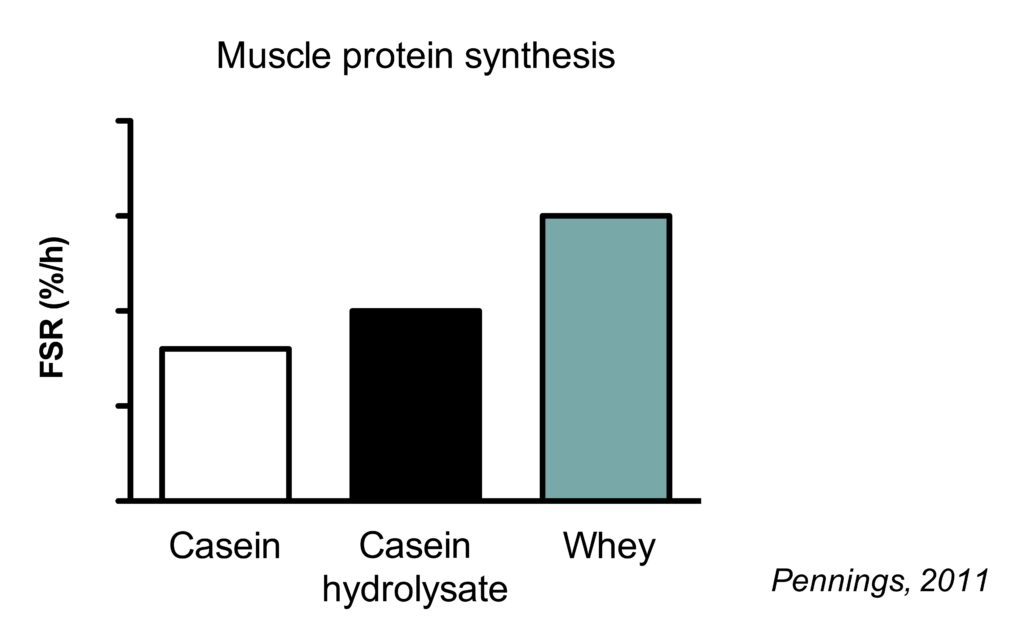
This is best illustrated by study which compared the muscle protein synthetic response to casein, casein hydrolysate and whey protein.
Casein is a slowly digesting protein. When intact casein is hydrolyzed (chemically cut into smaller pieces), it resembles the digestion of a fast-digesting protein. Consequently, hydrolyzed casein results in higher MPS rates than intact casein.
However, the muscle protein synthetic response to hydrolyzed is lower than that of whey protein. While both proteins are fast digesting, whey protein has a higher essential amino acid content (including leucine) (Pennings, 2011).
Animal based protein sources are typically have a high essential amino acid content and appears more potent than plant protein to stimulate MPS (Van Vliet, 2015). However, there this can potentially compensated by ingesting a greater amount of plant protein (Gorissen, 2016).
7.3 Leucine
Leucine is the amino acid that is thought to be most potent at stimulating MPS. Peak plasma leucine concentrations following protein ingestion typically correlate with muscle protein synthesis rates (Pennings, 2011). This supports the notion that protein digestion rate and protein leucine content are important predictors for anabolic effect of a protein source.
While leucine is very important, other amino acids also play a role,
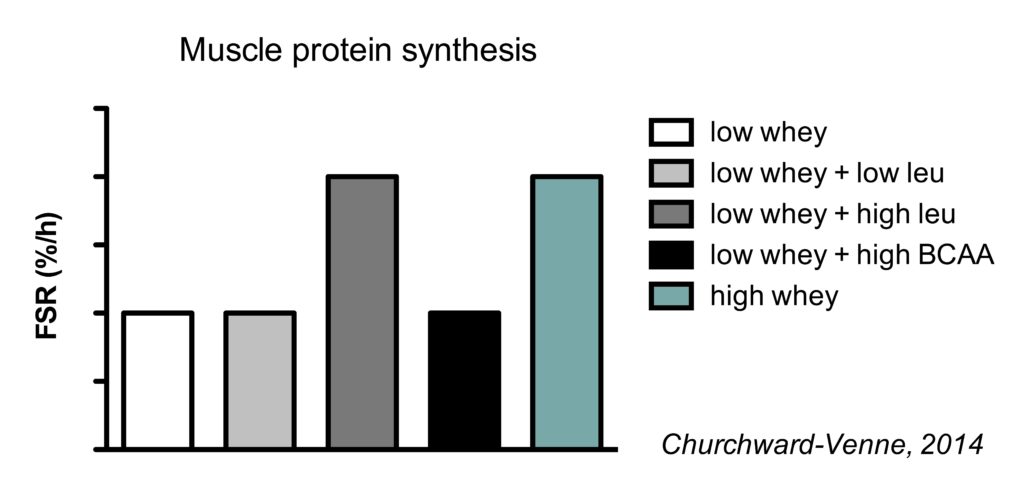
This is best illustrated by study (Churchward-Venne, 2014) which compared the muscle protein synthetic response to five different supplemental protocol:
- 6.25 g whey
- 6.25 g whey with 2.25 g leucine for a total of 3 g leucine
- 6.25 g whey with 4.25 g leucine added for a total of 5 g leucine
- 6.25 g whey with 6 g BCAA added (4.25 g leucine, 1.38 g isoluecine for, and 1.35 g valine)
- 25 g whey (contains a total of 3 g leucine)
All five conditions increased muscle protein synthesis rates compared to fasting conditions. As expected from our earlier discussion on the optimal amount of protein, 25 gram of protein increased MPS rates more than just 6.25 gram.
Interestingly, the addition of 2.25 gram leucine to 6.25 gram whey did not further improve MPS. Since this low whey + low leucine amount had the total amount of leucine as 25 g whey (which contains 3 g leucine), it indicates that leucine alone doesn’t determine the muscle protein synthesis response.
The addition of a larger amount of leucine (4.25 gram) to the 6.25 gram whey did further improve MPS, with MPS rates being similar to the 25 gram of whey. This indicates that the addition of a relatively small amount of leucine to a low dose of protein can be as effective as a much larger total amount of protein.
Finally, it’s really interesting that the addition of the 2 other branched-chain amino acids (BCAA) appeared to prevent the positive effect of leucine on MPS. Isoleucine and valine use the same transporter for uptake in the gut as leucine. Therefore, it is speculated that isoleucine and valine compete for uptake with leucine, resulting in a less rapid leucine peak which is thought to be an important determinant of MPS rates.
7.4 Carbohydrate or fat co-ingestion
Carbohydrates slows down protein digestion, but have no effect on MPS (Gorissen, 2014). In agreement, adding large amounts of carbohydrates to protein does not improve post-exercise MPS rates (Koopman, 2007).
In addition to the effects on protein digestion, it has been suggested that carbohydrates stimulate insulin release, which may stimulate muscle protein synthesis and/or muscle protein breakdown rates. However, the addition of carbohydrates to post-exercise protein has no effect on muscle protein synthesis or breakdown rates. The effects of insulin on muscle protein breakdown rates are described in more detail in section 2, and the effects of insulin on muscle protein synthesis are further described in section 7.6.
Adding oil to protein does not slow down protein digestion or MPS (Gorissen, 2015). It possible that oil simply floats on top of a protein shake in the stomach, and that a solid fat would delay digestion. One study has reported a greater increase in net muscle balance following full-fat milk compared to fat-free milk (although this study used the 2 pool arterio-venous model which is not the most reliable measurement).
7.5 Whole food and mixed meals
Most research has looked at isolated protein supplements in liquid form such as whey and casein shakes.
Thirty gram protein provided in the form of 113 gram 90% lean grounded beef results in a similar MPS response as 90 gram protein (340 gram beef). This supports the protein dose-response relationship observed with protein supplements where 20 g of protein gives a near maximal increase in MPS.
Minced beef is more rapidly digested than beef steak, indicating that food texture impacts protein digestion. However, there was no difference in MPS between these protein sources.
Beef protein is more rapidly digested than milk protein. However, milk protein stimulated MPS more than beef in the 2 hours (Burd, 2015). Between 2 and 5 hours, there was no significant difference between the sources. This indicates that digestion speed does not always predict the muscle protein synthetic response of a protein source.
As discussed in the previous section, the addition of carbohydrate powder or oil to a liquid protein shake does not impact muscle protein synthesis. However, it is unknown how the components of (large) mixed meals interact. For example, the addition whole-foods carbohydrates such rice, potatoes, or bread to whole-food protein sources such as chicken.
It can be speculated that the protein in mixed meals is less rapidly digested, which is typically (but not certainly not always) associated with a lower increase in MPS.
7.6 Insulin
As described in my systematic review, insulin does not stimulate MPS (Trommelen, 2015).
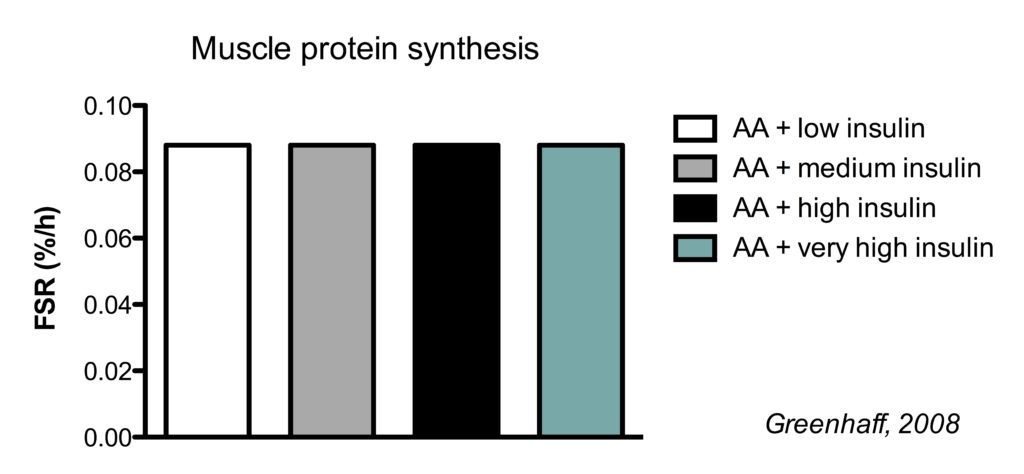
This is best illustrated by a study which clamped (maintained) insulin at different concentrations and also clamped amino acids at a high concentration.
There were four conditions:
- high amino acids + low insulin
- high amino acids + medium insulin
- high amino acids + high high insulin
- high amino acids + very high insulin
In the illustration below, you see the effects on muscle protein synthesis.
Regardless whether insulin levels were kept low (similar to fasted levels) or very high, MPS rates were the same in all conditions.
In my systematic review, I describe the effect of insulin in other conditions including in the absence of amino acid infusion, but the conclusion remains that insulin does not stimulate MPS under normal conditions (Trommelen, 2015). However, it should be noted that insulin stimulates MPS at at supraphysiological (above natural levels) doses (Hillier, 1998). In the bodybuilding world, insulin is sometimes injected at supraphysiological doses to stimulate muscle growth.
Insulin inhibits muscle protein breakdown a bit, but only a little is needed for the maximal effect (this is discussed in dept in section 2). A protein shake alone increases enough insulin to maximally inhibit muscle protein breakdown, you don’t need additional carbohydrates (Staples, 2011).
7.7 Protein timing
Exercise improves the muscle protein synthetic response to protein ingestion. Therefore, it has been suggested that protein intake immediately post-exercise is more anabolic than protein ingestion at different time points.
Probably the best evidence to support the concept of protein timing is a study which showed that protein ingestion immediately after exercise was more effective than protein ingestion 3 h post-exercise (though this study used the 2 pool arterio-venous method which is not a great measurement of muscle protein synthesis) (Levenhagen, 2001). In contrast, a different study observed no difference in MPS was found when essential amino acid were ingested 1 h or 3 h post-exercise (Rasmussen, 200).
In addition, resistance exercise enhances the muscle protein synthetic response to protein ingestion for at least 24 hour (Burd, 2011). It is certainly possible that the synergy between exercise and protein ingestion is the largest immediately post-exercise and then slowly declines in the next 24 h hour. However, these data suggest that there is not a limited window of opportunity during which protein is massively beneficial immediately post-exercise, that suddenly closes within a couple of hours.
Overal, no clear benefit to protein timing has been found in studies measuring muscle protein synthesis studies. As such studies are much more sensitive to detect potential anabolic effects compared to long-term studies measuring changes in muscle mass, it unlikely that long-term studies will observe benefits of protein timing.
In agreement, a meta-analysis concluded that protein supplementation ≤ 1 hour before and/or after resistance exercise improved muscle mass gains (Schoenfeld, 2013). However, this effect was largely explained by the fact that the protein supplementation increased total protein intake, rather than the specific timing of protein intake.
From a practical perspective, a protein shake during or after a workout:
- is easy to do
- theoretically may have a very small benefit
- is a simple method to improve total protein intake
- prevents FOMO (fear of missing out on something)
So it could be argued: why not do it? It is mostly up to personal preference.
7.8 Protein distribution
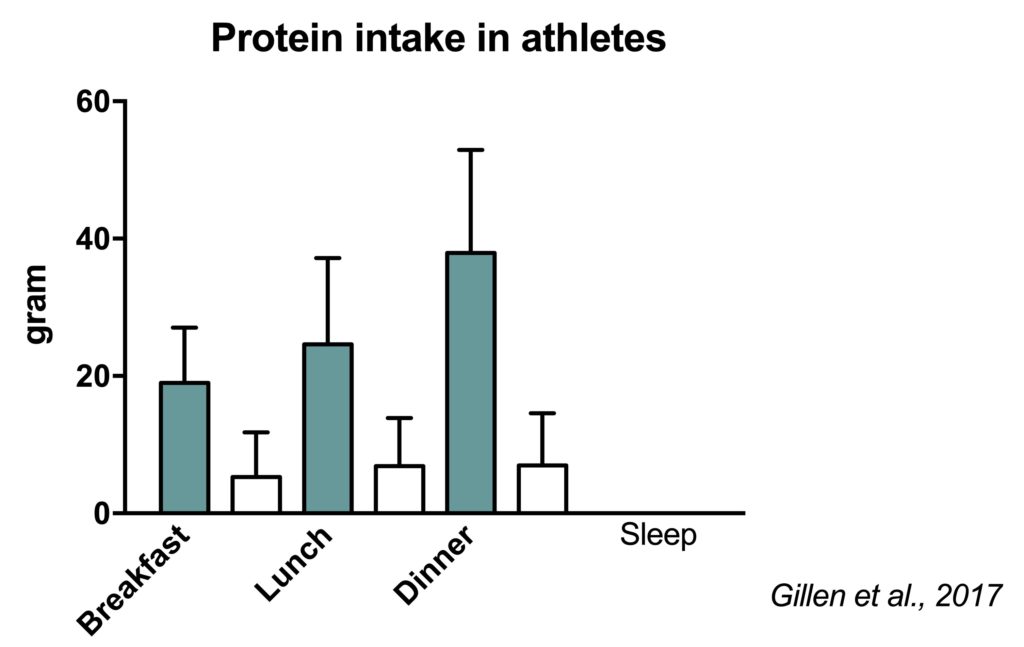
We performed a study to assess protein intake in well-trained Dutch athletes. Even some Olympic athletes were included. We observed that athletes consumed ~1.5 g protein/kg/d based on multiple dietary recalls (Gillen, 2017). However, their real protein intake is likely ~25% higher based on a nitrogen excretion validation study we performed in a subgroup (Wardenaar, 2015).
The majority of the protein was consumed in the three main meals: breakfast, lunch and dinner. While this intake pattern has a reasonable distribution throughout the waking hours, amino acid availability is potentially low during the night.
This begs the question: does protein distribution throughout the day matter for muscle protein synthesis?
Several studies suggest that protein should be reasonably distributed for optimal anabolism. For example, an even balance of protein intake at breakfast, lunch and dinner stimulates MPS more effective than eating the majority of daily protein during the evening meal (Marerow, 2014). But a too high distribution resulting in many mini snacks may also be suboptimal. Providing 20 g of protein every 3 hours stimulates MPS more than providing the same amount of protein in less regular doses (40 g every 6 hours), or more regular doses (10 g every 1.5 hour) (Areta, 2013).
While there are more studies that support the concept of protein distribution, there are even more studies that suggest it has no clear benefit. If your goal is to absolutely maximize gains, it theoretically makes sense to try to aim for at least a reasonable protein distribution (3-4 protein rich meals divided throughout the day). But the impact of protein distribution is likely going to be small at best and you definitely don’t have to set a timer for each meal.
PS: I have some new data on this topic that I can’t share yet….but….it’s exciting 😉
7.9 Muscle full effect
The muscle full effect is the observation that amino acids stimulate MPS for a short period, after which there is a refractory period where the muscle does not respond to amino acids. More specifically, after protein intake, there is an lag period of approximately 45-90 min before MPS goes up and peaks between 90-120 minutes, after which MPS returns rapidly to baseline even if amino acid levels are still elevated (Bohe, 2001)(Atherthon, 2010).
The muscle full effect has given birth to a theory on how to optimize protein intake throughout the day in the online fitness community. It suggests that after amino acids levels have been elevated, you should let them drop down back to fasting levels to sensitize the muscle to amino acids again. Subsequently, protein intake will stimulate MPS again.
There is no evidence to support this theory.
The suggested mechanism seems unlikely as many food patterns result in elevated amino acid levels throughout the whole day. The traditional bodybuilding diet consists of very frequent, very high protein meals (e.g. chicken, rice, broccoli 6 times a day). In fact, it was specifically designed with the goal of keeping amino acids elevated throughout the whole day so there would always be enough building blocks for form new muscle tissue. Or intermittent fasting where all daily protein is eaten in a short time period (usually 8 hours).
These diets would only allow for a single ~90 min increase in MPS during a whole day. Clearly, that’s not what’s happening as these approaches are used by many with great success to improve muscle mass.
More likely, there’s a refractory period and it simply takes some time before the muscle responds to AA again. You don’t have to let amino acids drop to sensitize the muscle.
However, the relevance of the muscle full effect can be questioned in athletes.
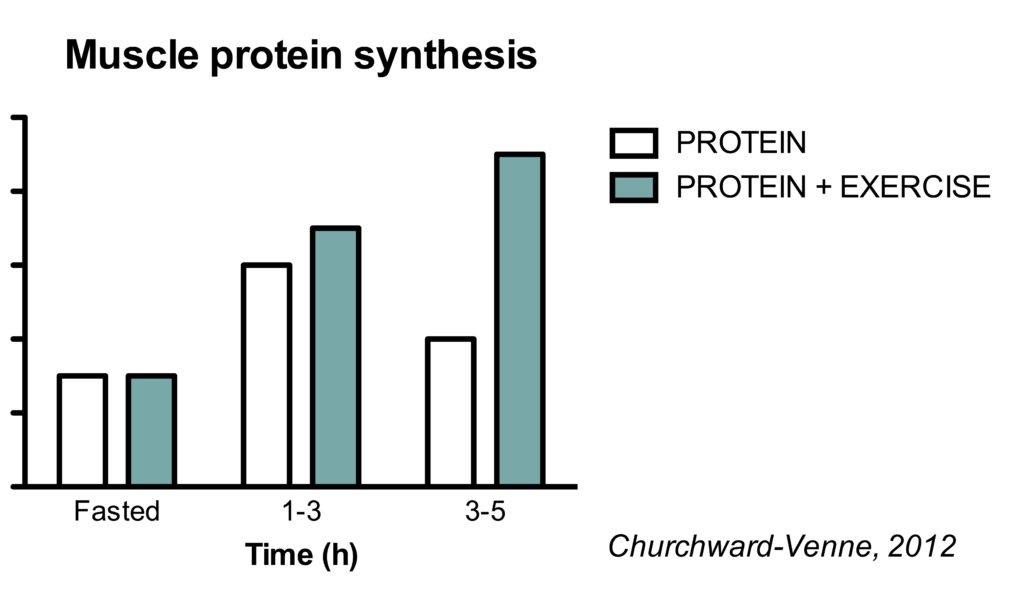
This is best illustrated in a study where the effect of protein was assessed in both rested and post-exercise conditions (Churchward-Venne, 2012).
Protein intake alone stimulates MPS in the 1-3 h period after ingestion. Subsequently, MPS rates fall back to basal rates. However, in post-exercise condition, protein stimulated MPS rates in both the 1-3 h and the 3-5 h period.
It appears that the muscle full effect is not present in acute post-exercise conditions. It’s interesting to note that resistance exercise can increase the sensitivity of the muscle to protein for at least 24 h (Burd, 2011).
The muscle full effect is an interesting phenomenon, but we don’t understand it well enough to let it influence our protein intake recommendations.
7.10 Protein prior to sleep
As discussed above, an effective protein distribution optimizes MPS. Therefore, it makes sense to eat protein just prior to overnight sleep, the longest period you can’t eat. 40 g of protein prior to sleep increases MPS during overnight sleep (Res, 2012). Protein supplementation (27.5 g) prior to sleep during a 12-week resistance training program improves muscle mass and strength gains (Snijders, 2015).
7.11 Energy intake
Only three days of dieting already reduce basal MPS (Areta, 2014). This shows that an energy deficit is suboptimal for MPS, however you can grow muscle mass while losing fat (Longland, 2016). It is unclear if eating above maintenance is needed to optimize MPS.
8. Summary
- While muscle protein breakdown is an important process, it doesn’t fluctuate much, which makes it far less important for muscle gains than muscle protein synthesis
- Whole-body protein synthesis is not really relevant for athletes. (often just called protein synthesis in studies, don’t confuse it with muscle protein synthesis)
- Muscle protein synthesis is predictive for muscle hypertrophy.
- Muscle protein synthesis studies are more sensitive to pick up anabolic effects than long-term studies measuring changes in muscle mass
- It’s easy to draw wrong conclusions if you don’t fully understand the methods.
How to optimize muscle protein synthesis: exercise guidelines:
- Train each muscle group at least twice a week with multiple sets
- Rep range doesn’t matter if you train (close?) to failure
- Rest at least 2 minutes between sets
How to optimize muscle protein synthesis: nutrition guidelines:
- Eat 4-5 meals spread throughout the day: e.g. breakfast, lunch, post-workout shake, dinner, and pre-sleep.
- Eat 20-40 g protein at each meal. Amounts above 20 g give a small additional benefit.
- Choose animal protein (whey protein is the best). Or compensate by eating larger amount of plant protein.
- If your main goal is to build muscle, eat at least maintenance calories.
Now I need your help.
Please bookmark this post for a couple of reasons:
First, I will keep this post updated with new research.
Second, I will continue to further elaborate sections based on your feedback and add additional sections in the future.
Lastly, please reference specific sections from this article when you see a discussion on muscle protein synthesis. People mistaking whole-body protein synthesis for muscle protein synthesis: see section 4.1. Someone skeptical about a conclusion from a paper because muscle protein breakdown was not measured? Section 2 buddy. Someone claiming that protein supplementation is not effective based on a long-term study he read that found no improvement in muscle mass: section 5 got you covered.
Feel free to ask me questions about the methods, or interpretation on protein metabolism studies in comments or on Facebook. If certain questions keep popping up I’ll rewrite the related sections to make it more clear for everyone.



![1-[13C]-leucine](http://www.nutritiontactics.com/wp-content/uploads/2016/04/1-13C-leucine.jpg)



I stabled up on this post a few years back. I just want to thank you and tell you how much i appreciated this article. It is very informative and to the point.Helps you understand the science behind. You really have done a tremendous job explaining everything
Nice to see you come back to the article after several years.
I will slowly start updating it, so hopefully I see you in the comments again in the future!
This is the holy grail of protein synthesis white papers/articles. Many of us have seen the musclebound cow with the genetically engineered lower myostatin. Is there anything out there that practically works for lowering myostatin to your knowledge?
Thank you Ray!
Unfortunately no practical way of inhibiting myostatin (exercise does it a bit but ofcourse not as extreme as the cow mutation).
To be fair, myostatin is not all bad. There is evidence that myostatin deficiency increases changes on injuries, because your other tissues (for example tendons) can’t keep up with your muscle strength. So in practice you might want to lower it a bit, but not turn it completely off.
I have read some articles on pubmed about gut muscle axis ,what is your take, could the increased need of protein to stimulate MPS in elders have something to do with change in gut microbiota , according to articles i read it seems possible but, it seems kind of little research
Possible, but very speculative at this point.
The microbiota are implicated in just about anything, but usually there is little evidence to support it and/or little you can do about it in practice.
You recommended 4 to 5 meals, however in another part of the article you said that 3 to 4 meals should be enough. Is there significant difference between eating 3 or 4 meals a day to maximize MPS?
As we and others have done more and more research on this topic, the support for a high meal frequency has become weaker and weaker.
See here my recent thoughts on protein distrubtion:
https://www.nutritiontactics.com/protein-intake-distribution-beneficial-detrimental-or-inconsequential-for-muscle-anabolism/
A-MA-ZING and highly instructive article, plus a tremendous work to aggregate and synthetize all this data!!! Thanks a lot, Jorn.
With these new information in mind I’m confronted with a dilemma though. I’ve been practicing intermittent fasting for 30 years or so, which has been a blessing for my physical and intellectual energy and my overall health: no breakfast, 30 min callisthenics or HIIT workout every morning, light meal (fruits + nuts) round 2PM and main food intakes in the 5-9PM window, including a moderate amount of protein foods (typically 3 eggs or 200 g white meat or fish, + 200 g quinoa or buckwheat, + 5 g of BCAA 4.1.1 on callisthenics days).
That’s a rough estimate of 80 g of proteins per day – without taking into account the other protein sources like vegetables, which seem pretty negligible to me(?).
For my 77 kg of body weight (slim figure), that’s significantly less than the 120 g you recommend.
I’ve read on several studies that it’s useless to take more than 30 g of proteins PER MEAL (the excess is not assimilated), so I don’t see how to raise my protein intake to 120 g/day while keeping my intermittent fasting rhythm!?…
Any thoughts and/or suggestions?
Thank you Gilles.
I certainly have some thoughts!
We have shown that there is no limit to how much protein you can absorb or use for muscle growh in a meal:
https://www.nutritiontactics.com/greater-protein-dose-prolongs-anabolism/
This may seem in contrast to other studies, but the difference can easily be explained: higher doses of protein take more time to do their magic. If you don’t give the protein that time in a study, you miss the effect and draw the wrong conclusion.
And see here for some more thoughts on protein distribution:
https://www.nutritiontactics.com/protein-intake-distribution-beneficial-detrimental-or-inconsequential-for-muscle-anabolism/
Thank you for these two enlightening articles of yours about the prolonged anabolism in case of a unique daily protein intake. (Sorry for my late reply: I’ve happened to re-read your main article “by accident”. Too bad there doesn’t seem to be automatic e-mail notifications of replies, I almost missed yours!…)
You could include https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164527/
Pre-Sleep Consumption of Casein and Whey Protein: Effects on Morning Metabolism and Resistance Exercise Performance in Active Women
That study does not measure muscle protein synthesis.
But we have done a pre-sleep whey vs casein MPS study:
https://pubmed.ncbi.nlm.nih.gov/36857005/
I will include it in the post once I find some time.
Absolutely amazing treatise on the topic! Thankyou!!
I had a question: the Burd study you cited showed good evidence for three sets of resistance exercise being superior to one set for muscle protein synthesis both in anmplitude and duration. Are you aware of any other studies that show whether there is an upper limit or a likely level of diminishing returns for this? General advice from coaches/bodybuilders anecdotally suggests it’s around 6 sets per muscle group per workout, but I can’t find any studies to support the idea.
It would be good to know if there’s a) an upper limit (presumably further sets beyond this are “wasted”) and b) what the relationship is like ie doing double the work only yields 20% more MPS then it might not be appropriate for people looking to get the best bang for buck so to speak. This would also give sone guidance as to determining absolute weekly volume and potential frequency of training.
Thanks again for the great post.
Thank you Greg.
Unfortunately, very little studies are done on volume and the MPS response. It’s a tricky topic for MPS studies, because it seems likely that muscle protein breakdown goes up with a higher volume. In the article, I discussed that in most circumstances muscle protein breakdown is a non-factor that you can ignore, but for different training programs with unequal volume it may be a factor that needs to be taken into account (which is difficult).
There is some research from longer-term studies on muscle mass and training volume (although those have there own challenges). When I last looked at the literature, there seemed to be a consensus that you want to hit at least 10 sets per week, with potentially small upside going higher than that but probably not a good bang for buck.
This is guesswork, but I can imagine your training volume (expressed as sets taken close to failure) needs to increase over the years are your training status increases. But such work of different training experiences is completely lacking.
I love your article and I have a little question below:
If I work out 3 days a week (e.g. Tuesday, Thursday, Sunday) and am looking to do a lean bulk, would you suggest ensuring there is more of a surplus the day of exercises and the following day (during heightened protein synthesis) than for example on Saturday when I would have had 2 days rest from the gym?
To clarify, I am assuming that on Saturday, there would not be much protein synthesis occurring from the Thursday workout, so if I were to have a surplus, would it be better to eat more on the Thursday and Friday and possibly to a maintenance calorie day on Saturday to limit fat when muscle gain is not likely to happen?
Thanks!
Energy balance on the short term does not seem to impact muscle protein synthesis. Your body sort of keeps track of the last couple of days (and longer), rather than just the moment.
Also the increase in energy expenditure from training/increased MPS is quite small.
You could play around with your strategy. But I think in practice it’s much easier to keep your caloric intake the same between days.
Hi Jorn, are there any papers/articles that cover this idea that energy balance the days before a workout impact MPS? I tend to eat in a calorie surplus the 24 hours post workout but from what you’ve said it would be more beneficial to be in a surplus the 24 hours before a workout?
Hi Olly,
I don’t think you can “trick” you body by calorie cycling. Your body takes your energy intake over a period of days into account.
What food is the highest source of leucine for vegan bodybuilders to consume right after weight training? Tnx
Corn protein probably has the highest leucine content per g of protein.
I’m a 37 year old female. Do you know if there is any difference in MPS between males and females? I’m looking to get as strong as possible.
There’s no real difference between young males and females in protein metabolism.
Females tend to start with lower muscle mass than males. But relative growth is the same and there is no clear indication that protein requirements are different.
After reading your guidelines they don’t seem to fit in with the Leucine threshold of 2.5g per meal which requires near 30g protein per meal. Maybe the Leucine threshold theory is weak?
Leucine is an important amino acid. But the leucine threshold concept is not the ideal basis to determine your protein intake.
I bookmarked this page and read down and see the author asking me to bookmark the page, I rarely bookmark pages but this one is very interesting . Good job. this page doesn’t contain floating annoying adds. I think should add the alcohol negative effect and also caffeine that can alter sleep. I have heard that sleep is important I don’t know.
Thank you!
The article focuses on the two main impacts on MPS: exercise and protein ingestion. I didn’t want to keep adding more and more. But yeah, high doses of alcohol and sleep restriction are detrimental for MPS. We have infographics + short articles on the site on both!
I read:
1. Bio-availability for MPS varies by the protein source, i.e., veggie less efficacious than animal, and therefore 30%-ish more veggie protein needed.
2. Protein powder brands, like the Walmart Six Star was less absorbable / useful, than say, Optimum Nutrition.
I didn’t know if the latter was based on a well done / generally accepted study, or was it more marketing based.
Am I to presume the protein numbers you cited above come from meat / dairy – just so I can calibrate (I’m a carnivore).
Lastly, if I need to consume 25% more Six Star protein powder to be equally bio-available to the ON brand, first it appears to negate buying the cheaper powder, but is it possibly also a bit of a waste on my body to consume more of something less useful?
I know that last one goes beyond the scope of the MPS topic we’re on.
Thanks Jorn
1. Yes, there is some indication for this. I would not say you definitely need x% more. But if you want to play it safe with plant-based protein, it’s better to take a bit more.
2. I googled the Six Starts but couldn’t quickly find the ingredient list. But it seems to be whey protein, so there should not be much difference with Optimum Nutrition whey protein.
3. Yes, numbers in the article are based on high-quality protein.
I love this topic! Please help me reconcile this disparity. How do I, a 187lb man (85kg), get my daily recommended 1.6-2.0g /kg (136-170g), or the often touted 1g per 1lb of body weight (185g / d), IF I’m aiming for minimum effective dose – eating 20g / m every 3 hours for 16 hours (sleeping 8hrs per night). That’s only 100g / d. 3 meals of 40g / d, is only 120g / d. Am I to eat 136g-185g / d, assuming the excess goes to other bodily needs, and know the max my muscles can use are ‘roughly’ the lower numbers above?
Hi Chandler.
20 g is considered an “optimal” dose, because higher doses have relatively little added value. But they do have some value.
Athletes tend to eat about 35 g protein in dinner, so there is no need to tell them to reduce that.
We also recommend at least 40 g protein prior to sleep, because that protein needs to last you the whole night.
When you take those numbers into account, it falls nicely in line with recommendations.
If you’re looking for minimum effective dose, really you just aught to eat whatever, whenever, but train well. Training stimulus matters significantly more than the nutrition aspect, and one could build muscle with 60g/day of bean protein if they’re eating enough calories otherwise.
Newer study came out showing that 100g of milk protein stimulated muscle synthesis for much longer than 30g due to short digestion, so that explains why timing matters very little overall.
Thanks for sharing the informative article.
In 6.4 it talks about intranet rest periods, does this apply just to working a specific muscle group? I typically pair push and pull exercise or different groups to give longer break between hitting a muscle group. I guess my question is, does the whole body need 3 to 5 minutes rest or just the specific muscle group being worked?
Yes the resting periods are for specific muscle groups.
A-MA-ZING and highly instructive article, plus a tremendous work to aggregate and synthetize all this data!!! Thanks a lot, Jorn.
With these new information in mind I’m confronted with a dilemma though. I do intermittent fasting for 30 years or so, which has been a blessing for my physical and intellectual energy and my overall health: no breakfast, 30 min callisthenics or HIIT workout every other day in the morning, light fruit meal round 2 PM and main food intakes in the 5-9 PM window, including a moderate amount of protein foods (typically: 100 g nuts or 3 eggs or 200 g white meat or fish). That’s a rough estimate of 30 g of proteins per day – without taking into account the other protein sources like vegetables, which seem pretty negligible to me(?).
For my 78 kg of body weight (slim figure), that’s A LOT LESS than the 120 g you recommend.
1) I’ve observed that my body doesn’t tolerate high doses of proteins (say: one meat dish for 3-4 days in a row): it triggers throat infection + fever + flu-like symptoms for 1-2 days, sometimes swollen joints… I don’t know exactly what it means about my metabolism but my “digestive comfort zone” seems to be around the 30 g/day I mentioned. What do you (and science) think about this?
2) Even if I decided to raise my protein intake to 120 g/day, I don’t see how that would be compatible with my intermittent fasting rhythm!?… I’m planning on including 1 or 2 doses of BCAA during the day before my evening meal but it would barely fill the gap towards 120 g! Any suggestions?
Hi Gilles,
Thank you for the kind words. I should have a new publication later this month that ties in a bit with intermittent fasting. Once that’s out, we can discuss the topic in more detail!
Hello Jorn,
I can’t find your December article about intermittent fasting, please…
I have questions to ask you:
If MPS is optimally stimulated in a 24hours period after exercise, why not training every day each muscle group for optimal gains?
Is getting a pump for a long time (like a full hour, by stimulating the muscle multiple times to stay in that state) will lead to more nucleus overload and/or hypertrophy than hitting the muscle for a single, high intensity, “destroying” set ?
1) The exact timeline of MPS will depend on many things, including training status and the intensity and volume of the training. You would also have to take recovery into account.
2) no
You write: “bigger dudes don’t need more protein to get the same response as smaller guys.” And certainly, the linked study shows that the MPS response to protein is not affected by LBM.
But what about MPB, whose response is mediated by “increas[ing] the insulin concentration?” Does the 25g dose of whey protein increase the insulin concentration for a 105kg athlete as much as it increases the insulin concentration for a 70kg athlete? If not, might the 105kg athlete receive an MPB benefit from adding carbohydrate to the pre-workout meal, even if the 70kg athlete would receive no such benefit? Is there anything definitive on this? Or, in the absence of anything definitive, do you have any practical advice for larger men? (I don’t particularly like pre-workout carbs, but if adding a modest amount will benefit me with respect to MPB, it wouldn’t be a big deal to throw some in.)
No there’s nothing definitive on it. But I wouldn’t worry about MPB. MPB is something you need for optimal adaptation. Don’t think of it as bad.
Alcohol consumption has been shown to reduce muscle synthesis. But those studies primarily looked at alcohol consumption immediately after exercise. Some of the figures you reference show peaks in muscle synthesis after only a few hours, but elsewhere you say the peak is at 24 hours. So if alcohol consumption comes 10-12 hours after exercise, how much muscle synthesis are you really losing out on?
The timeline/peak of the MPS response appears to depend on many variables such as the volume of the training and training status. In general, don’t think timing is something that makes a lot of difference. So specifically for alcohol: I don’t think trying to strategically time your alcohol is going to help much. It’s going to reduce recovery/gains.
i eat one time a day and follow a ketogenic diet.
i train in the evening fasted state.
Then i get 20 gr EAA.
Then i have dinner around 8-10 PM with lean meat/fish/chicken and vegetables.
How much eaa or high quality amino acid should i take and when to maximize my muscle gain/muscle protein synthesis?
Thank you
What time is that 20 g EAA exactly? That is a pretty good dose, that should maximize your anabolism until your dinner.
Thank you for your answer. On training days, As soon as I finish my workout, I consume 20 gr EAA. On non-training days, I am also planning to take 20 gr EAA with meal after my 24 fasting. I am 43, so in order to Increase my satellite cell count I try to induce autophagy.
“ PS: I have some new data on this topic that I can’t share yet….but….it’s exciting”
When can we expect this new information? 🙂
Unfortunately will take quite a while (like 6-24 months, because the processes of submitting, peer review (sometimes they ask additional experiments…) is so slow.
Not even a hint? If there’s no benefit to protien timing and you can eat it all in one go, it would be nice to know I’m not wasting my time. 😛
Though I don’t think it’s massively important, I expect synthesis is still available for longer in higher doses of protein after exercise..
Hi Mika,
The study has been out now for a couple of months:
https://www.nutritiontactics.com/greater-protein-dose-prolongs-anabolism/
Short version:
– you don’t waste protein if you eat a lot in a meal
– protein distribution probably likely has little to no impact on muscle mass
Hi Jorn,
Excellent article, well summarized.
I was looking into the effects of BCAAs vs BCAA + EAA vs Whey supplementation after fasting. Some of the studies referenced touched on it a bit, but wasn’t the focus.
I was wondering if there was a meta analysis done or consensus on training post-fast with AA supplementation.
There is no meta-analysis.
The results would be whey protein > BCAA + EAA > BCAA.
Whey protein is complete (provides everything that you need)
BCAA+EAA are complete, but the low NEAA requires some of the EAA to be converted. Does not have to be a problem if the dose is high enough
BCAA alone: suboptimal. Does not provide all EAA.
Hi do older males need more protein to get the same effect as younger males
Yes, see https://www.nutritiontactics.com/elderly-need-more-protein-for-muscle-growth/
Amazing article, really helpful thank you for that.
You don’t cover the daily recommended/optimal total proteins intake though, the section of “amount of protein” suggest a dosage per meal and not per weight.
Do you have any informations in that regard ?
1.6-2.0 g/kg/d if you want a simple guideline.
“Casein is a slowly digesting protein. When intact casein is hydrolysed (chemically cut into smaller smaller pieces), it resembles the digestion of a fast digesting protein. Consequently, HYDROLYZED WHEY results higher MPS rates than intact casein.”
From the context I assume this should be HYDROLYZED CASEIN, right? 😀
Yes, you’re right. Changed it now. Thanks!